A Scientist’s Quest to Save Her Husband’s Life Using Phages
It was November 2015 in Egypt. Two scientists, Steffanie Strathdee and her husband, Tom Patterson, had just returned to their holiday cruise and enjoyed an evening meal after a long day of visiting the pyramids. That’s when Patterson fell ill. He brushed it away, suspecting it was a case of food poisoning that would go away after taking some standard antibiotics.
However, the symptoms worsened. At first, it was nausea. Then, there was pain in his abdomen and his back. Soon after, he was diagnosed with gallstone pancreatitis, which is when a gallstone blocks one’s pancreatic duct, causing inflammation and pain. However, little did the couple know that these symptoms were only the beginning.
The couple’s vacation was put to an end as Patterson had to be evacuated to Germany. Soon enough, it became clear that he was facing a serious health problem, but dose after dose of antibiotics made no difference to his recovery - his health only worsened and the reason for this was antibiotic resistance.
Antibiotic resistance occurs when bacteria develop mutations that allow them to defend against antibiotic medication, making it very difficult for physicians to treat bacterial infections in humans.
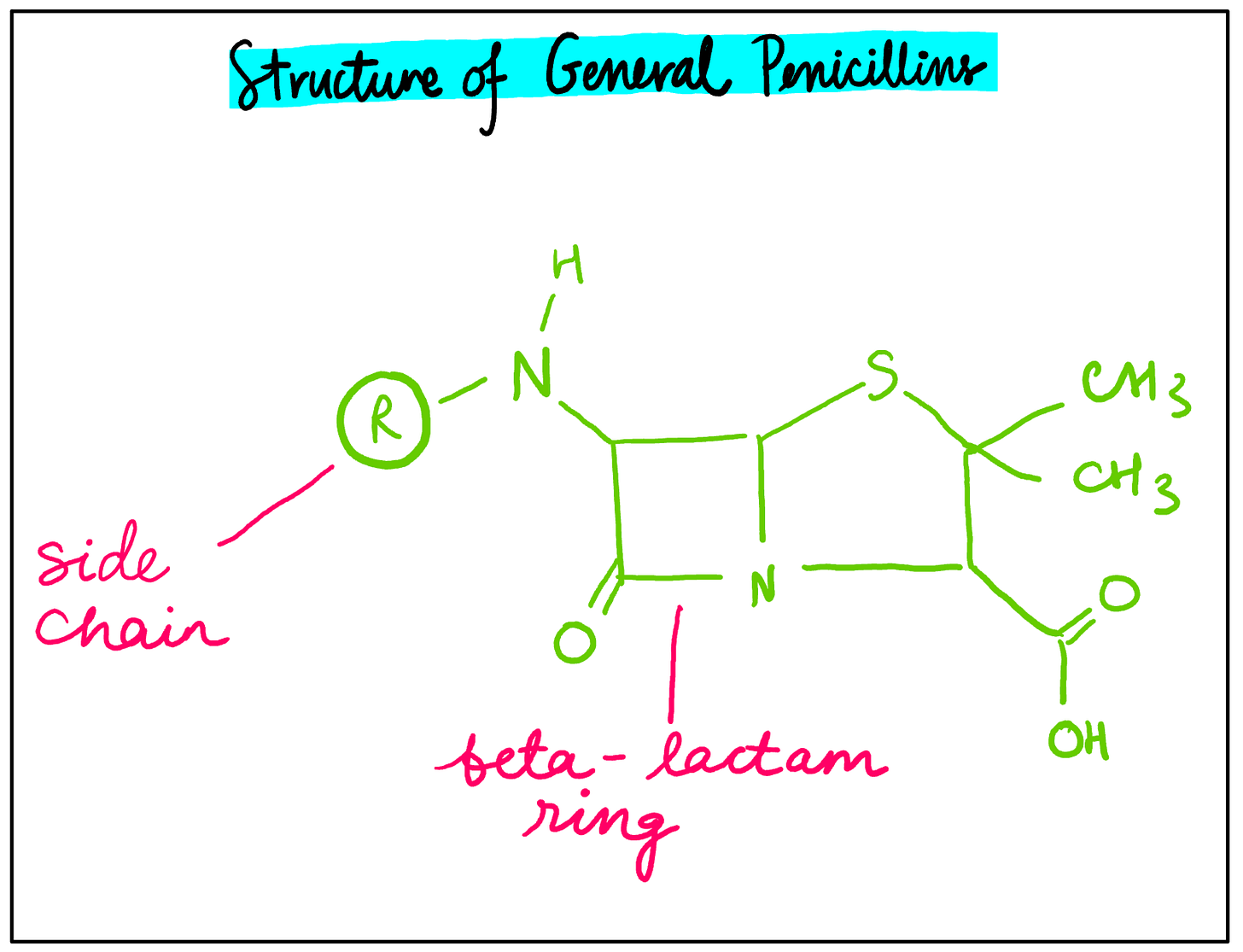
Penicillin is an example of one of the most common types of antibiotics. Two key structural features of general penicillins include a beta-lactam ring as well as a side chain which varies depending on the type of penicillin (see figure 1). The bond angles of the beta-lactam ring are 90° each, which causes ring strain and makes the ring very reactive. When penicillin is administered to a patient, the beta-lactam ring breaks open and binds to the enzyme transpeptidase, preventing cross-linking within bacterial cell walls. This makes the cell walls weaker and more permeable to water, which causes the bacterial cells to fill up with water and burst.
Figure 1: A diagram illustrating the chemical structure of general penicillins.
However, one way in which certain bacteria have developed resistance to penicillin is through genetic mutations which increase the production of the enzyme penicillinase. This causes the enzymatic breakdown of penicillin and renders it useless for treating infections.
Although Patterson had been prescribed several antibiotics, none of them had worked. Doctors soon identified the culprit behind his illness - a superbug. This is a type of bacteria that is highly resistant to multiple antibiotic drugs. The superbug strain that was affecting Patterson was growing in a large pseudocyst or a collection of leaked pancreatic fluids near his pancreas. Six of the most dangerous superbugs can be remembered using the acronym ESKAPE, and Patterson was affected by the ‘A’ pathogen - Acinetobacter baumannii or A. baumannii.
Superbugs like A. baumannii are dangerous because they often use more than one mechanism of antibiotic resistance. For instance, they can change the permeability of their cell membrane to prevent antibiotics from entering the cell. They can also form clusters known as biofilms (see figure 2), where they surround themselves with a layer called an extracellular polymeric substance matrix. This surrounding layer protects the bacteria from an antibiotic attack in a similar way a walled fortress would protect an army from a blitz.
Figure 2: A rough sketch illustrating the process of biofilm formation.
When the superbug was discovered to be growing uncontrollably in Patterson’s body, he was flown home to San Diego, where, after several months of failed antibiotic treatments, he became weaker by the day. The infection was spreading throughout his body and his organs were beginning to fail. He had several cases of septic shock and hallucinations as he lay on the hospital bed in a coma. Hoping to find a solution, his wife, Stephanie Strathdee, a scientist and professor at the University of California San Diego, began to research other treatments online.
She found several research studies of viruses known as bacteriophages (see figure 3) or ‘bacteria-eaters’ which had been used in the Soviet Union to treat bacterial infections, but little information on their use in the Western world. With the rise of antibiotic successes, research into bacteriophages, also known as phages, had historically taken a backseat in scientific communities across the West, especially in the US. While there are case studies of phage therapy in the US, they are less frequent and considered to be less reliable since they have not undergone systematic clinical trials across larger populations.
Nevertheless, Strathdee gathered keen fellow scientists from around the world in an attempt to make phage therapy a reality for her husband. She knew that the journey would not be easy. Scientists have estimated that there are about 1031 phages on the planet, and they are found practically everywhere, including the soil, in oceans, and inside organisms.
Normally, phage therapies utilise lytic phages, which enter the host bacterial cell, make copies of themselves, and then cause the bacterial cell to burst and die. This helps the phage release its copies to kill other bacteria.
However, phages selectively target bacteria. Therefore, despite their abundance, a phage and its corresponding bacterium need to be matched precisely, otherwise the phage therapy will not work. Strathdee and the team members supporting her were faced with an overwhelming task: finding a phage that would match the culprit of her husband’s infection.
Figure 3: An annotated sketch illustrating the general structure of a phage.
With the help of the hospital at the University of California San Diego, PubMed, and a team of expert scientists from around the world, Strathdee embarked on a tough research journey. Luckily, she had access to research from various labs that had gathered large collections of phage samples. These phage samples were purified before being tested.
Bacterial isolates or samples were then collected from the pancreatic drainage in Patterson’s body, and mixtures of phages were tested on these isolates. During the research process, almost 200 lytic phages specific to A. baumannii were used in mixtures to test against the bacterial isolates. This was because prior research had often shown that multiple phages delivered collectively could lead to a more significant outcome than a single type of phage.
From the hundreds of phages screened in the labs, only a few had a visible effect. An assay system was used to discern the amount of time these phages were able to inhibit bacterial growth. Some of the identified phages were able to inhibit bacterial growth for over 20 hours! However, the bacterial isolate resisted against the first two phage mixtures, and it was the third phage mixture that was administered to Patterson.
However, prior to administration, there were several purification processes that needed to be carried out to ensure that the phage mixture would meet FDA standards. For instance, the mixture had to be diluted in sodium lactate solution to prevent endotoxins released by bacterial host cells from damaging the patient’s body cells after the administration of the phage mixture.
In March 2016, the phage mixture was intravenously administered to Patterson, who was on the verge of death. However, 3 days later, he awoke from his coma and was able to talk for the first time in weeks. Over the following three weeks, the phage mixture was delivered regularly, and an antibiotic called minocycline was later added to the dosage when researchers found that certain A. baumannii samples were vulnerable to it. For about two more months, Patterson stayed in the hospital, receiving phage therapy and showing signs of improvement throughout his stay. After 245 days in the hospital, he was finally discharged and soon enough, was healthy enough to return to his job!
Patterson was the first person in the US to receive phage therapy for treating a multi-drug-resistant bacterial infection, and Strathdee’s persistence led to an inspiring medical breakthrough in the fight against antibiotic resistance. Antibiotics have become some of the most commonly used methods to treat bacterial infections. However, antibiotic resistance is becoming a growing issue due to the overprescription and overuse of antibiotics, especially in healthcare and agriculture. Bacteria are gaining regular exposure to these drugs, which increases the likelihood of them developing resistance to multiple antibiotics and turning into superbugs like A. baumannii.
Lytic phages, which are very specific about the bacterial strains that they target, are difficult to process and isolate because of their overwhelming abundance in the environment. However, if the scientific community continues to add newly identified phages to existing phage libraries, phage therapy could start to be utilised more frequently.
The specificity of phages may even become a significant advantage because this would prevent them from killing beneficial bacteria in the human body, which is a problem that several antibiotics can cause. Furthermore, antibiotics take years to develop, whereas preparing a phage mixture for therapy may only take a few months if the required phage has already been found. Phage therapy could therefore become an important method for tackling the superbug crisis alongside the use of antibiotics.
Strathdee’s quest to save her husband’s life using phage therapy is not only an incredible story of love and resilience in trying times, but also a testament to the strength that scientific communities can acquire when they experiment with new or even obscure research opportunities and work collaboratively towards a common goal.